From Beginner to Expert: Modeling Medical Knowledge into General LLMs (2312.01040v3)
Abstract: Recently, LLM based AI systems have demonstrated remarkable capabilities in natural language understanding and generation. However, these models face a significant challenge when it comes to sensitive applications, such as reasoning over medical knowledge and answering medical questions in a physician-like manner. Prior studies attempted to overcome this challenge by increasing the model size (>100B) to learn more general medical knowledge, while there is still room for improvement in LLMs with smaller-scale model sizes (<100B). In this work, we start from a pre-trained general LLM model (AntGLM-10B) and fine-tune it from a medical beginner towards a medical expert (called AntGLM-Med-10B), which leverages a 3-stage optimization procedure, i.e., general medical knowledge injection, medical domain instruction tuning, and specific medical task adaptation. Our contributions are threefold: (1) We specifically investigate how to adapt a pre-trained general LLM in medical domain, especially for a specific medical task. (2) We collect and construct large-scale medical datasets for each stage of the optimization process. These datasets encompass various data types and tasks, such as question-answering, medical reasoning, multi-choice questions, and medical conversations. (3) Specifically for multi-choice questions in the medical domain, we propose a novel Verification-of-Choice approach for prompting engineering, which significantly enhances the reasoning ability of LLMs. Remarkably, by combining the above approaches, our AntGLM-Med-10B model can outperform the most of LLMs on PubMedQA, including both general and medical LLMs, even when these LLMs have larger model size.
Sponsor
Paper Prompts
Sign up for free to create and run prompts on this paper using GPT-5.
Top Community Prompts
Explain it Like I'm 14
What this paper is about (big picture)
This paper shows how to teach a general-purpose AI LLM to become much better at medical questions. The team starts with a regular LLM called AntGLM-10B and turns it into a medical specialist called AntGLM-Med-10B. They do this in three steps that are a lot like school: first the model “reads” medical knowledge, then it practices following medical instructions, and finally it trains hard on a specific kind of medical test. The goal is to make a smaller model (10 billion parameters) perform like a medical expert, even compared to larger models.
What the researchers wanted to find out
Here are the main questions they asked, in simple terms:
- How can we turn a general AI into a medical expert without making it huge?
- What training steps help most for medical skills: general reading, instruction practice, or focused test prep?
- Can smart prompting (like checking each multiple-choice option carefully) make the AI reason better on medical exams?
- Can a smaller, well-trained model beat bigger ones on tough medical benchmarks (like PubMedQA)?
How they trained the model (the approach, explained simply)
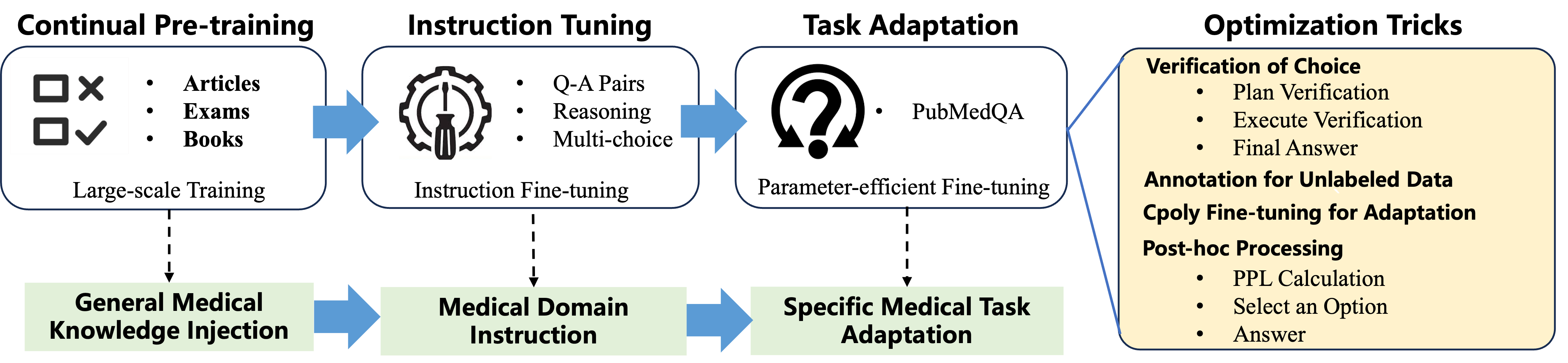
Think of the model as a student going from beginner to expert. The team used a three-stage plan:
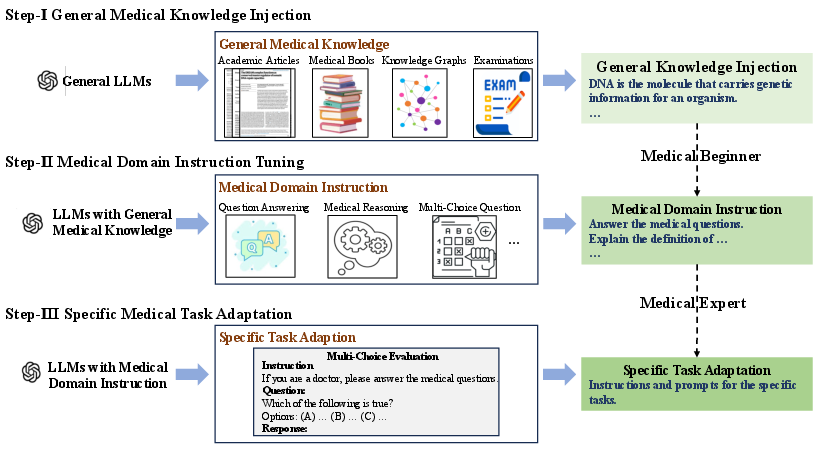
- Stage 1: General Medical Knowledge Injection
- Analogy: “Reading the textbook.”
- The model keeps pre-training on lots of medical text to learn the basics. This included:
- Medical books (clear, structured knowledge)
- Knowledge graphs (like a big map of medical facts turned into sentences)
- Medical Q&A pairs (real consultations)
- Exam questions (converted into readable facts)
- Medical articles (like PubMed abstracts and science articles)
- Goal: Build a strong medical foundation.
- Stage 2: Medical Domain Instruction Tuning
- Analogy: “Practicing with a teacher’s instructions.”
- The model learns to follow medical task instructions across many formats:
- Answering questions, reasoning, multiple-choice, and conversations
- This helps it understand how to respond properly to different medical prompts.
- Stage 3: Specific Medical Task Adaptation
- Analogy: “Focused test prep for a particular exam.”
- They target multiple-choice medical questions, especially the PubMedQA test (questions about medical research papers that have answers like yes/no/maybe).
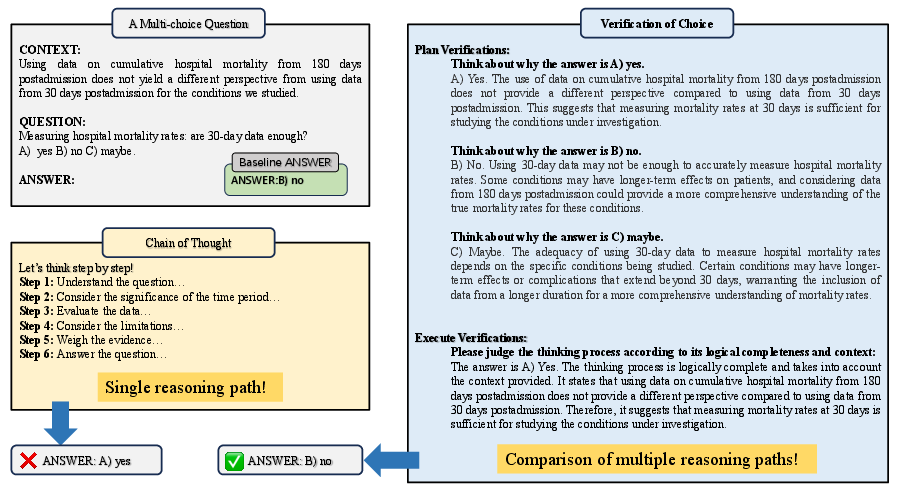
- They introduce a new prompting method called Verification-of-Choice (VoC):
- CoT (Chain-of-Thought) is like “show your work” for one answer.
- CoVE (Chain-of-Verification) is like “double-check your answer.”
- VoC goes further for multiple-choice: the model writes its reasoning for each choice (A, B, C), compares them, spots mistakes or mismatches, and then picks the best one. It’s like a student explaining every option before selecting the answer.
- They also use:
- LoRA/adapters: think “plug-in skills” that are small add-ons instead of retraining the whole brain, making training faster and cheaper.
- C-Poly (multi-task adapters): like having shared skill modules plus task-specific modules so the model can learn common tricks across tasks and still specialize.
- Perplexity ranking: a “how surprised am I?” score the model uses to pick the most likely choice.
They also cleverly labeled unlabeled training data (PQA-U) by having the model generate answers using VoC—like making “practice tests” with answer keys to learn from.
What they found and why it matters
Here are the standout results:
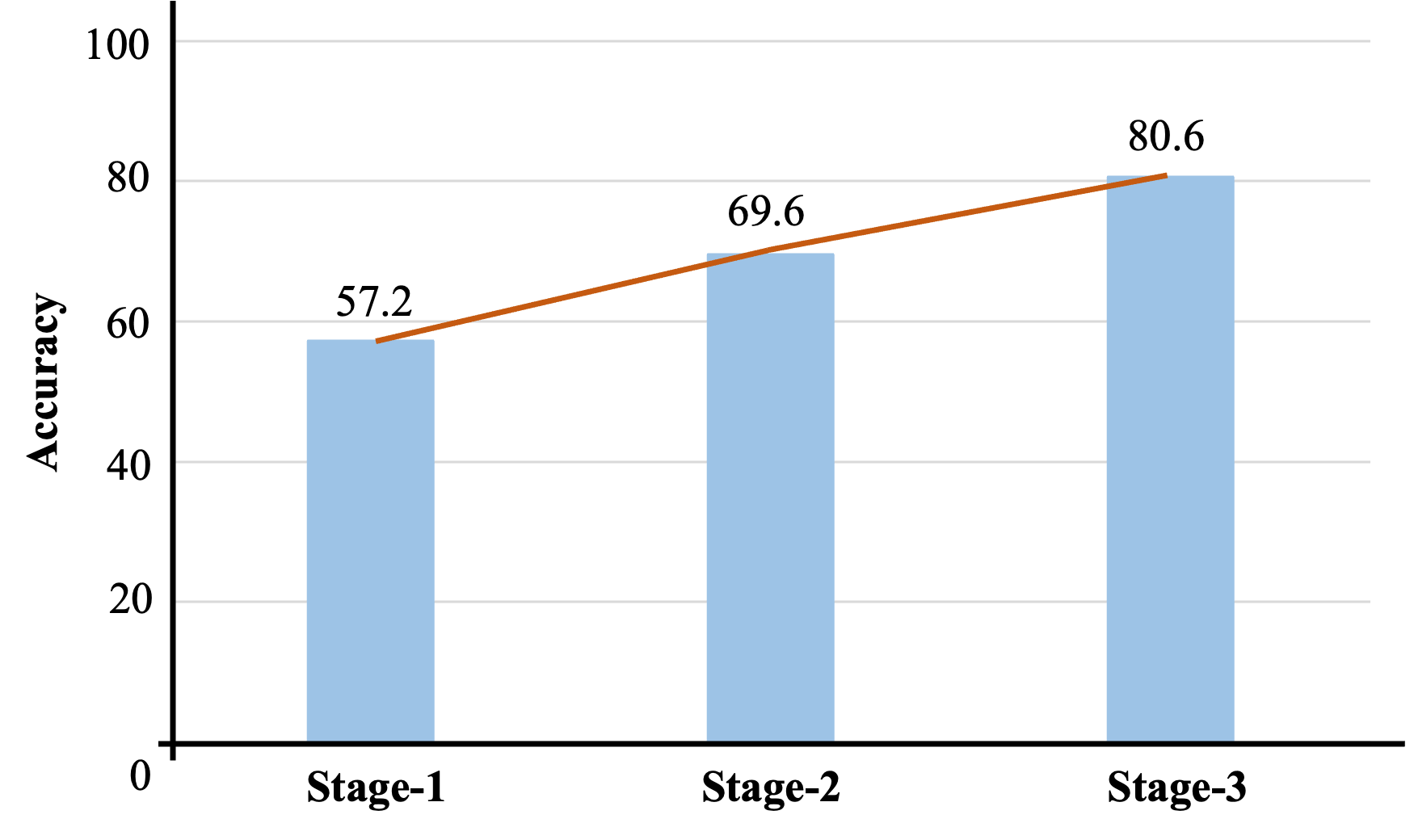
- Big accuracy jump across stages:
- Before any medical training, the model scored about 57.2% on PubMedQA.
- After the full three-stage process, it reached 80.6%.
- This shows each step added real value, especially the final, focused adaptation.
- Competitive performance with a smaller model:
- AntGLM-Med-10B (10B parameters) scored 80.6% on PubMedQA.
- That’s close to the very best models (like Med-PaLM 2 at 81.8%) and better than many larger models.
- Lesson: Smart training can beat raw size.
- Verification-of-Choice helps:
- Using VoC to label the unlabeled data improved results further.
- In short, explaining and checking each option before choosing boosts reasoning accuracy.
- Multi-task training with adapters (C-Poly) works well:
- Training on several related datasets together helped more than training on one at a time.
- Shared “common skills” plus “task-specific skills” made learning more efficient.
Why this is important: It shows you don’t need the biggest model to get expert-level performance if you train cleverly. This can make high-quality medical AI more accessible and efficient.
What this could mean for the future
- Better medical AI with fewer resources: Hospitals, clinics, and researchers could run strong models without always needing giant, expensive systems.
- Safer, more reliable reasoning: Techniques like Verification-of-Choice encourage the model to think carefully, not just guess—useful in sensitive fields like medicine.
- A roadmap for other expert fields: The same three-stage approach (read widely, practice instructions, then specialize) could help build expert AIs in law, finance, or engineering.
- Still, use with care: Even with strong results, medical AI should support—not replace—health professionals. Testing, supervision, and ethics remain crucial.
In short, this paper presents a smart training plan that turns a general AI into a capable medical assistant, showing that better “paper habits” can rival sheer size.
Knowledge Gaps
Knowledge gaps, limitations, and open questions
Below is a concise list of what remains missing, uncertain, or unexplored in the paper, written to be concrete and actionable for future research.
- Evaluation scope is narrow:
- Results focus primarily on PubMedQA; comprehensive benchmarks (e.g., MedQA-USMLE, MedMCQA, MMLU-Clinical, MultiMedQA) are not reported with standard splits, hindering claims about broad medical competence.
- No real-world, clinician-authored, or workflow-oriented evaluations (e.g., case vignettes, differential diagnosis, triage, guideline adherence).
- Reproducibility and evaluation protocol clarity:
- Inconsistent PubMedQA results (80.6% “main result” vs. 88%+ in fine-tuning table) with unclear splits, seeds, and whether the official test set and evaluation server were used.
- Several ablations use 500-sample subsets without specifying sampling protocols, seeds, or whether they match standard dev/test splits.
- Lack of full details on prompt templates, hyperparameters per stage, early stopping criteria, and selection of best checkpoints.
- Potential data contamination and leakage:
- PubMed abstracts are used for pretraining; only PQA-L abstracts are excluded, but possible overlaps with other PubMedQA subsets or evaluation-like data are not systematically deduplicated or reported.
- PQA-U pseudo-labels are generated using the dataset’s “long answers,” which may encode answer cues; deduplication and leakage audits (hashing, n-gram overlap, semantic similarity) are not provided.
- Quality and ethics of training data:
- Synthetic rewrites (knowledge graphs, exam questions) lack quality control studies (factual accuracy, consistency, bias).
- “Purchased professional medical articles” introduce licensing, usage rights, and provenance questions; no licensing or ethical review details provided.
- Real-world QA pairs may contain sensitive information; privacy protection, de-identification, and IRB/ethics approvals are not discussed.
- Cross-lingual generalization is untested:
- Training leverages extensive Chinese and English data, but performance on Chinese medical benchmarks (e.g., CMB, C-Eval medical tracks, CMExam, Huatuo-related test suites) is not reported, leaving bilingual transfer unknown.
- Generalization beyond multiple-choice is unproven:
- The approach and VoC prompting are tailored to multiple-choice; effectiveness on open-ended, long-form, or evidence-grounded medical QA (e.g., justification with citations) remains untested.
- No evaluation on tasks like summarization of clinical notes, guideline recommendation synthesis, or SOAP note generation.
- Verification-of-Choice (VoC) analysis is limited:
- No direct comparison with strong reasoning baselines (self-consistency, majority vote over CoT samples, debate, verifiers/critics, PoT/program-of-thought, tool-augmented verification).
- Computational overhead (latency, token budget) of VoC is not quantified; cost–benefit trade-offs are unknown.
- VoC’s robustness with >3 options, longer contexts, and adversarial distractors is untested.
- No analysis of VoC failure modes (e.g., confirmation bias, inconsistent rationales).
- Hallucination and faithfulness not rigorously measured:
- Claims about reduced hallucination via verification are not supported by standardized metrics (e.g., fact-score, attribution fidelity) or third-party fact-checking benchmarks.
- No tests of rationale faithfulness (e.g., causal scrubbing, input perturbation studies) for CoT/VoC explanations.
- Uncertainty and calibration remain open:
- Perplexity-based selection is used ad hoc without calibration evaluation (ECE, Brier score), selective prediction, or abstention strategies for safety-critical settings.
- Safety, harm, and bias:
- No safety alignment (RLHF with clinical constraints, constitutional health policies), bias/disparity audits, or toxicity/unsafe advice evaluations.
- No adverse event simulation or harm-reduction protocols; no mechanism for refusal/triage when uncertain.
- Catastrophic forgetting and retained general abilities:
- The impact of medical specialization on general-domain capabilities is unmeasured (pre/post comparisons on general benchmarks like MMLU, HELM, BIG-Bench, or GLUE/SuperGLUE).
- Multi-task adapter method (C-Poly) limitations:
- Authors note that C-Poly’s router “cannot index and effectively predict untrained unknown tasks”; generalization and out-of-distribution task routing remain unresolved.
- No comparison with alternative MTL/PEFT methods (e.g., mixture-of-adapters, prompt-tuning, IA3, (IA)3, LoRA variants with routing, sparsely gated MoE) under equal compute.
- Scaling and data ablations are missing:
- No systematic scaling paper across model sizes (e.g., 7B/13B/34B) or data sizes per corpus; contributions of each corpus (books, KGs, exams, QA, articles) to final performance remain opaque.
- No paper of continual pretraining duration vs. gains or instruction-tuning mixture composition and sampling strategies.
- Training efficiency and environmental impact:
- Wall-clock time, total tokens seen, compute budget, and energy usage are not reported; implications for reproducibility and sustainability are unclear.
- Error analysis is absent:
- No breakdown by question type (causal, statistical, trial design), linguistic phenomena (negation, numerical reasoning), clinical domain (cardio, oncology), or class (yes/no/maybe) to guide targeted improvements.
- Robustness and security:
- No robustness evaluation against noisy abstracts, contradicting evidence, prompt injection, or distribution shifts (e.g., newer PubMed years, non-PubMed sources).
- Retrieval and tool augmentation:
- The approach is purely parametric; benefits of retrieval-augmented generation (e.g., PubMed search with grounding and citation), tool use (calculators, guidelines), or external verifiers are unexplored.
- Deployment constraints:
- Inference latency, memory footprint, and throughput—especially with VoC—are not quantified; suitability for clinical settings with time constraints is unknown.
- Transparency and release:
- It is unclear whether the model, prompts, and curated datasets (with licenses) will be released; lack of artifacts limits independent verification and broader impact.
- Pseudo-labeling of PQA-U remains uncertain:
- No human auditing of pseudo-label accuracy or estimated noise rate; no confidence-thresholding, self-training iterations, or co-teaching strategies to mitigate label noise.
- Outdated or incomplete baselines:
- Comparisons exclude several recent open-source medical LLMs (e.g., LLaMA-2/3-based med models, BioGPT-XL, ClinicalCamel, PMC-LLaMA, MedAlpaca variants), limiting the strength of claims about competitiveness.
- Clinical integration and governance:
- No discussion of how the system would integrate with clinical workflows, EHRs, audit trails, or regulatory requirements (e.g., ISO/IEC, FDA/EMA guidance for clinical AI).
Collections
Sign up for free to add this paper to one or more collections.